- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
- Services
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
Preclinical Blog
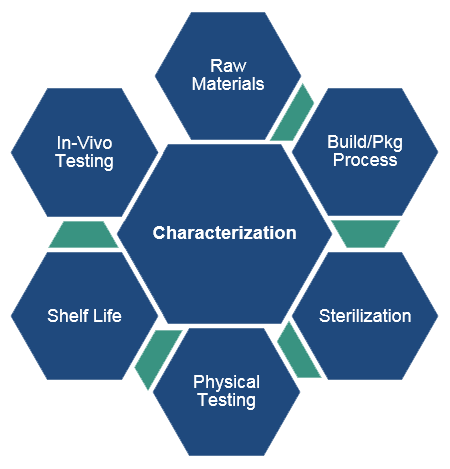
Test Article Characterization for Combination Product & Medical Device GLP Preclinical Studies
Nov 10, 2014
When planning an FDA GLP preclinical study for a medical device or combination product, it is important to consider the strategy for dealing with test and control article characterization early in the process, to ensure appropriate documentation of article build, sterilization, and testing. The following are general principles and commonly asked questions for test and control article characterization in a GLP preclinical study.
Why is characterization required for combination product or medical device test and control articles used in GLP preclinical studies?
- Reconstruction of the article
- Evidence of conformity to outlined specifications
- Demonstrated suitability of the article for the intended use and within the study application
What is characterization for a GLP preclinical study?
Characterization includes the material attributes of the medical device or combination product along with demonstrated functional attributes (often referred to as "stability"). Although they define different properties of the article, characterization and stability are often referred to collectively as "characterization."
Characterization = Basic Attributes
- Name and descriptors (e.g., medical device dimensions, drug concentration)
- Material composition (e.g., base material, overlay materials, coatings, radiopaque markers)
- If the article is or contains a drug or chemical component, the attributes of the substance must also be outlined (e.g., concentration, distribution, uniformity)
- Packaging
- Sterilization (e.g., method, location, and date of sterilization)
- Testing/inspection (e.g., any inspections or testing of the article attributes to assure that specifications were met)
- Hardware/software components must also be characterized and should be consistent through the study (e.g., avoid installing a new version of software partway through the study)
- Characterization documentation must be provided for each unique lot or batch of articles UNLESS it is a commercially marketed article in its commercial packaging (if it is a commercial article in full commercial packaging, no additional characterization documentation is needed)
- Characterization documentation should include sufficient information to support the article description contained in the study protocol, and traceable to the lots used in the study
Stability = Structural Stability Material or Substance Longevity
- Structural stability testing differs greatly, depending on the article
- Destructive testing of structural strength or stability
- Chemical testing (e.g. elution or absorption rates, leaching in a preservative/suspension)
- Impact of sterilization on materials or substances (e.g. breakdown of components, pre and post-sterilization drug coating analysis)
- Longevity under conditions of use (e.g. breakdown of materials in-vivo (particulation, resorption, device breakage)
- Material or substance longevity in defined storage conditions (basic shelf life / expiration)
- Stability must be documented for each article batch or construct (utilizing the same base materials), but stability testing may often be applied across lots
- Stability can be determined prior to the study, concurrently with the study, or some attributes may be assessed as part of the study (e.g. material breakage or particulation)
- Any stability assessment during the study should be performed equally on the test and control articles even if the article is a commercial article (the only stability information available on a commercial article is the expiration date and FDA has indicated that this is not sufficient for assessing article stability under study conditions)
How can combination product and medical device test article characterization be documented?
- The Sponsor can supply test and control article characterization information for a GLP preclinical study in many different formats and this information will be appended to the Surpass final report:
- Full manufacturing and testing documentation (Design History File, Device History Record, etc.)
- A signed, dated summary of manufacturing and testing, with reference to the appropriate master documents and their location(s) - note: Surpass can provide a template characterization memo for Sponsor convenience
- If the Sponsor is not able to perform testing under GLP compliance, the characterization information should specify the regulations/standards/data integrity steps that were followed, so that this can be noted in the Surpass final report
- The regulations require that characterization be provided for the final study report; however, Surpass recommends that this information be provided early in the process so that we can work together to answer any questions that may be needed for the study
Exact combination product and medical device characterization requirements can vary based on specific test and control article, so work with your trusted preclinical partner Surpass to identify the needs for your upcoming study. Give us a call, we're here to support you through the entire GLP preclinical study process. We will be posting additional entries in our blog with useful information for planning a GLP Study, so stay tuned.
Next Steps
- Sign up to receive notification of future blog posts by Surpass.
- View other blog posts related to Planning a GLP Study.
- Learn more about Surpass' GLP preclinical testing services.
- Contact us to learn more about medical device, pharmaceutical, biotech, or combination product GLP preclinical studies.
- Download our current Whitepaper on Preclinical In Vivo Study Design.




